Our Technology
Pioneering Gene Therapy for Rare Neurogenetic Diseases
Gene Therapy Approach
Innovative Technologies Driving Transformative Treatments
At NeuroGT, our mission is to harness the power of gene therapy to create life-changing treatments for patients with rare neurogenetic diseases. Our work centers on cutting-edge adeno-associated virus (AAV) vector-based therapies, which enable precise delivery of therapeutic genes to target cells. This approach addresses the root causes of diseases, offering hope where traditional treatments have fallen short.
Our research focuses on developing gene therapies for lysosomal storage disorders, such as Mucopolysaccharidoses (MPS) types I, II, and III. These conditions often lead to severe neurodegeneration and premature death. By delivering functional genes to the central nervous system, we aim to halt and slow disease progression, offering patients life-saving treatment and improved quality of life.
Our Focus: Addressing rare and devastating lysosomal storage disorders (LSDs)
MPS I
Hurler Syndrome
MPS I (Hurler syndrome, Hurler/Scheie syndrome) is caused by defect in the gene for iduronidase (IDUA), with estimated prevalence of 1:100,000 births
MPS I I
Hunter Syndrome
MPS II (Hunter Syndrome) is caused by the deficiency in the gene for iduronate-2-sulfatase (IDS). The disorder occurs in approximately 1:100,000 to 1:170,000 male births.
MPS IIIA, IIIB, IIIC
Sanfilippo Syndrome
MPS IIIA
MPS IIIA is caused by defects in the gene for heparan N-sulfatase (SGSH), with estimated prevalence of 1:50,000 births or lower.
MPS IIIB
MPS IIIB is caused by defects in the gene for alpha-N-acetylglucosaminidase (NAGLU), with estimated prevalence of 1:125,000 births or lower.
MPS IIIC
MPS IIIC is an ultrarare disease, caused by defects in the gene for heparan-alpha-glucosaminide N-acetyltransferase (HGSNAT), with estimated prevalence of 1:1,000,000 births or lower.
These diseases pose immense challenges, with few or no effective treatments available. Our therapies are designed to address both the systemic and neurological symptoms of these conditions.
Driving Innovation Forward
Platform AAV Gene Therapy Technolgy
Crossing the blood-brain barrier (BBB) to deliver therapeutic genes directly to the central nervous system is a critical breakthrough for addressing neurodegenerative conditions. With our AAV9 vectors, we ensure that therapeutic genes reach both the brain and peripheral tissues, providing comprehensive treatment that targets the systemic and neurological symptoms of diseases.
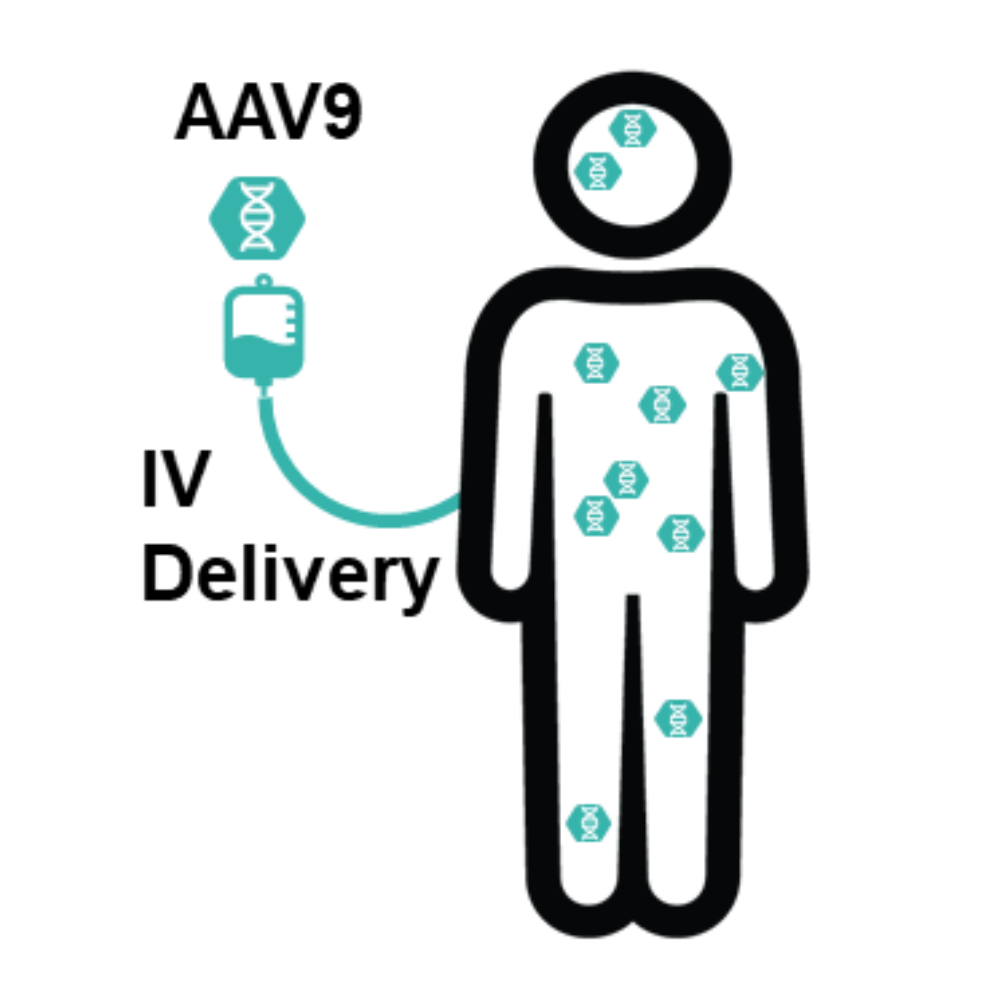
- NeuroGT utilizes AAV9 vectors that can cross the blood-brain barrier (BBB), allowing therapeutic genes to reach the central nervous system directly. This innovation is critical for neurodegenerative diseases, where traditional therapies often fail to address neurological symptoms.
- By delivering therapeutic genes to both the brain and peripheral tissues, we aim to halt or reverse disease progression and provide long-lasting benefits for patients.
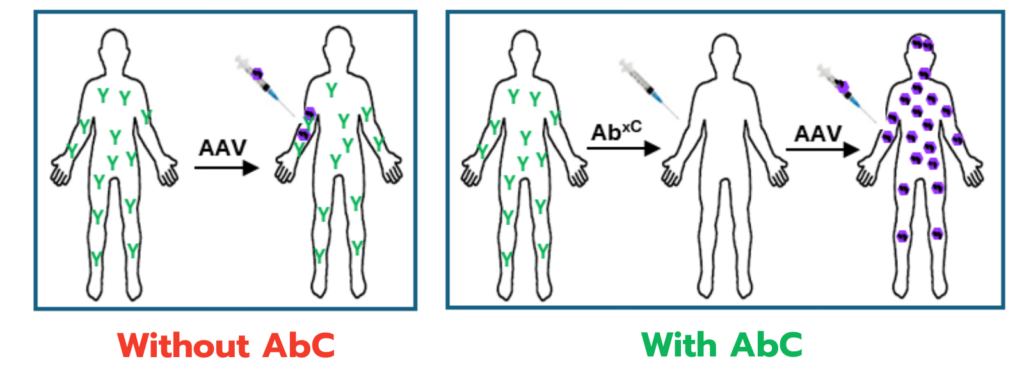
Antibody Cleaver (AbC)
One of the most significant barriers in gene therapy is the presence of pre-existing antibodies that can make patients ineligible for treatment or limit the possibility of redosing. Our proprietary AbC Technology overcomes this challenge by effectively clearing these antibodies, expanding treatment eligibility and allowing for repeat dosing when needed. This innovation opens the door to ongoing therapies for chronic conditions requiring multiple interventions.
AAV-Mediated EV-mRNA Cargo Technology
By leveraging extracellular vesicles to deliver mRNA, our AAV-mediated EV-mRNA Cargo Technology facilitates efficient protein production within target cells. This method not only enhances the efficacy of gene therapies but also improves safety by reducing the required dosage, ultimately broadening access to transformative treatments.
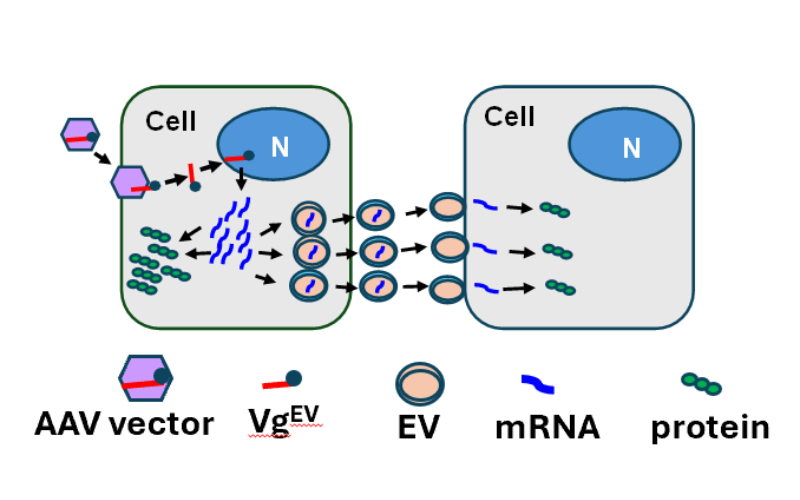
AAV-Mediated EV-mRNA Cargo for Bystander Effect
- For diseases involving non-secretory proteins, like certain forms of MPS, our AAV-Mediated EV-mRNA Cargo Technology facilitates a bystander effect, meaning that even cells not directly targeted by the therapy can benefit.
- This approach reduces the required dosage, increases safety, and ensures that therapeutic benefits extend throughout the affected tissues, addressing the widespread impact of neurodegenerative diseases.
Scale-up AAV Manufacturing Platform
NeuroGT uses state-of-the-art AAV manufacturing using a novel suspension cell line to support in-house development, and which can also be shared with external partners.
Our research is committed to transforming treatment outcomes for patients with neurodegenerative diseases, focusing on both the underlying biology and the practical barriers to effective gene therapy. Through these innovations, NeuroGT is setting a new standard for accessibility, safety, and long-term impact in neurogenetic therapies.
Research and Development
We take pride in our rigorous R&D process, which emphasizes:
Patient-Centric Design
Developing therapies tailored to patient needs and disease specifics.
Robust Preclinical Evaluation
Ensuring safety and efficacy through comprehensive studies.
Strategic Collaborations
Partnering with leading academic institutions and industry experts to accelerate innovation and bring therapies to market efficiently.
Our commitment to excellence and collaboration positions NeuroGT at the forefront of gene therapy advancements for neurogenetic diseases.
Research and Development
Advancing Progress in Gene Therapy
At NeuroGT, our R&D efforts reflect a rigorous, patient-centered approach to innovation. As a spinout from the University of North Carolina (UNC), we leverage cutting-edge research and collaborations to advance the field of gene therapy.
Every step of our R&D process is guided by the needs of patients and their families. From early research through clinical development, we focus on creating therapies that provide real, lasting impact for those with few or no current treatment options.
Our patient-first approach ensures that we are not only developing effective therapies but also addressing quality-of-life improvements for the individuals who need them most.
We are committed to a rigorous preclinical evaluation process, which helps us ensure safety and efficacy before advancing to clinical trials. By thoroughly assessing our therapies’ performance and potential, we build a solid foundation for success in clinical stages.
Our preclinical models allow us to test the therapeutic impact on both central and systemic symptoms, enabling comprehensive treatment solutions.
NeuroGT leads in developing advanced gene therapy platforms, such as our Antibody Cleaver (AbC) Technology and EV-mRNA Cargo Technology. These innovations are designed to overcome common barriers in gene therapy, such as immune response and efficient gene delivery, expanding access for more patients.
Our focus on platform technologies allows us to develop more effective, accessible therapies that address multiple neurogenetic conditions.
University of North Carolina (UNC) Partnership
As a UNC spinout, we maintain an extensive collaboration with the university, drawing on its wealth of expertise and resources in gene therapy research. This partnership accelerates our scientific advancements and strengthens our commitment to innovative, high-impact solutions.
Academic and Industry Partnerships
In addition to UNC, we collaborate with top academic institutions and industry leaders to support research, manufacturing, and clinical trial readiness. These collaborations enhance our ability to scale production and reach more patients, ensuring that our therapies have the potential for widespread impact.
Patient Advocacy Organizations
Our partnerships with patient advocacy groups inform our research priorities, helping us stay closely connected to the needs of the communities we serve. Through these collaborations, we work to align our innovations with patient expectations, ensuring that our therapies provide meaningful and transformative outcomes.
Through a robust and collaborative R&D process, NeuroGT is shaping the future of gene therapy for neurogenetic diseases. Our origins at UNC and our commitment to innovation, patient focus, and strategic partnerships allow us to make breakthroughs that matter, bringing us closer to a world where effective treatments for rare genetic disorders are within reach for all.
Other Research and Innovations
Expanding the Horizons of Gene Therapy
In addition to our core gene therapy programs, NeuroGT is advancing multiple innovative platforms and research initiatives to enhance the safety, accessibility, and effectiveness of gene therapy across a broader range of patients. Here’s what sets our research apart:
Scalable Suspension Cell Technology for AAV Vector Manufacturing
By streamlining the production process, we can meet the growing demand for gene therapy while ensuring quality and efficiency at every step.
Targeted Delivery Platforms
By improving delivery efficiency, especially to the central nervous system, our platform enables gene therapies to reach the specific areas impacted by neurodegenerative and neurogenetic diseases. By streamlining the production process, we can meet the growing demand for gene therapy.
Novel Biomarkers for Early Disease Detection and Monitoring
This focus allows us to develop therapies that not only treat the disease but also provide a clear way to measure progress, improving overall patient outcomes.

